| Home | |||||||||||
| |||||||||||
| Weitere Links: | |||||||||||
| | |||||||||||
Abstract
The isolated perfused bovine udder (Bovine Udder System, BUS-model) offers a scientific in-vitro method in the dermatological and cosmetic research as well. It provides information concerning substance interaction in natural skin and mucous membranes which can not be obtained with other in-vitro methods or in human studies. The review summarizes selected topics concerning the penetration patterns of vitamins (vitamin E, vitamin A palmitate, D-panthenol) and irritation potentials of surfactants (APG, SDS).
Introduction
The skin and mucous membranes are not just because of their anatomic and functional differences target organs of a particular type. In regular safety evaluations for occupational safety and consumer protection they are treated as possible zones of aggression for local or systematic harmful raw materials or chemicals. In cosmetics research the effectiveness of ingredients and formulations as well as the care of the skin and mucous membranes are of primary importance. The use of body care preparations can also be linked with the claim of providing protection against harmful environmental substances or of improving the status of the skin after the occurrence of exogenous or endogenous damage.
The apparently different demands placed an conventional and animal-free methods from the toxicological and cosmetic points of view are actually identical: the findings and results should allow as reliable an extrapolation as possible to the intended use an human beings (1).
With the implementation of the 6th amendment to the guidelines for cosmetics animal-free models have been additionally enhanced in status and have received considerable attention. It is no longer possible to imagine scientific literature without the results of countless research and validation projects designed for in-vitro studies. At the same time fundamental differences between the alternative and conventional test systems as well as misjudgements have become obvious; these have made the successful wide scale use of replacement methods either difficult or impossible (2, 3). In future the decision of the SCCNFP (Scientific Committee an Cosmetic Products and Non-Food-Products intended for Consumers) means that skin penetration as well as local tests an the skin and mucous membranes will become more important than they were previously (3).
The
Bovine Udder System (BUS) which was developed about 10 years ago for use in pharmacological
research (4) offers exemplary model conditions with the udder skin and the mucous
membrane of the teat cistern. Its intensive use in drug research has also led
to additional experience being accumulated about its predictability to human beings.
In scientific cooperation (Prof. Dr. M. Kietzmann, Veterinary College, Hanover)
this model arrangement has for several years been integrated into studies into
substance penetration and irritation in cosmetics research (5). In a similar way
other organs such as pig's ears or porcine legs have also been perfused and used
in dermatological research (6).
| Fig
1 a: Test set-up of the isolated perfused udder 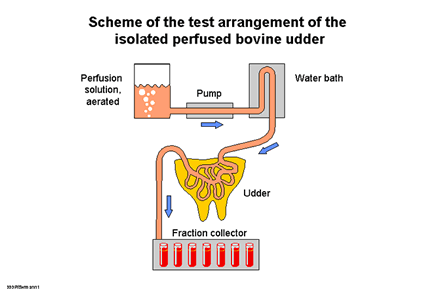 |
| 1
b) Various skin penetration phases 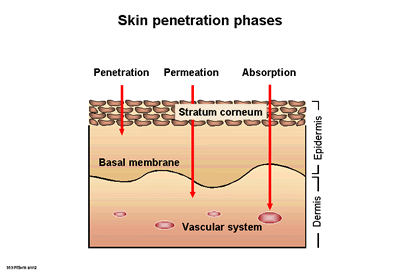
|
Principle
of the isolated perfused bovine udder
The
udder is pre-treated immediately after slaughter and connected to the perfusion
unit in the laboratory within 30 minutes without being refrigerated
(Fig. 1 a). Warmed, oxygen-enriched Tyrode solution is pumped into the venous
system at a rate of approx. 100 ml/minute at a constant pressure of 80-100 mm
Hg; each side of the udder is supplied separately. The continuous perfusion retains
the viability of the whole organ, i.e. the mammary gland tissue as well as the
skin and mucous membranes. Physical and biochemical tests are used to monitor
the viability of the organ including the skin and mucous membranes throughout
the whole perfusion period of (8 to 10 hours) without any further physical manipulation
(7).
| 1
c) Principle of the 'outside-inside' and 'inside-outside' concept 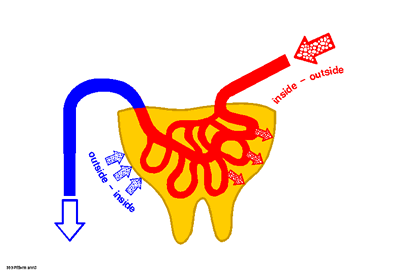
|
|
Fig.
(photo) 1: Outer udder skin 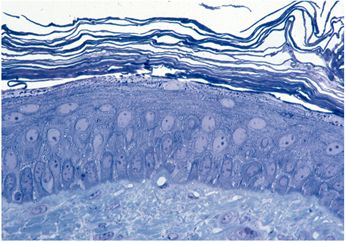
|
The skin of the udder (Fig. (photo) 1) is very fine, moderately hairy and possesses an epidermis and metabolism which are comparable to human skin together with a very thin, low-fat hypodermis and attached organs such as sebaceous and tubular glands. The mucous membranes of the cistern (Fig. (photo) 2) possess a two-row epithelium made of loose connective tissue and is accessible via the apical orificium (teat canal).
| Fig.
(photo) 2: Mucous membrane of the teat cistern 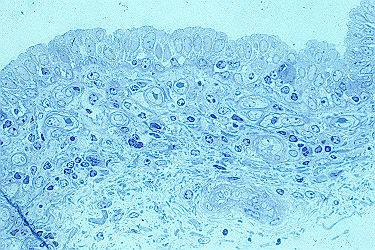
|
Application and exposure
As usual with the cosmetic application on skin or mucous membranes the test substances are applied topically according to the 'outside-inside' concept (Fig. 1 c). For studies an skin and mucous membranes in which the test substance is to be systematically applied via the vascular system the 'inside-outside' concept is used (8, 9).
In topical application either the open and/or occlusive one (Finn Chamber®, D = 18 mm) can be used under the normal cosmetic leave an (> 0.5 h exposure period) and/or rinse off (< 0.5 h exposure period) conditions, depending an the study design required. For detection of the substance in the context of penetration studies adhesive tape strippings of the horny layer are routinely used, as are dermatome sections (skin/mucous membrane sections 20 µm thick) or, if necessary, the perfusate can also be used by the means of a fraction sampler (Fig. 1 a). Time-dependent studies are easy to carry out because of the size of the application area. These samples and punched biopsies (whole skin / mucous membrane, D = 6 mm) required for the irritation study are removed after exposure times which differ in length. Owing to the vast amount of biological samples it is not necessary to use radioactively marked material for analytical purposes, while non-radioactive isotope marking is still possible (10).
Penetration into the skin and mucous membranes
In
contrast to pharmacological research, in cosmetic studies (topical application)
the penetration of ingredients into the stratum corneum or permeation into the
deeper layers of the epidermis or dermis are of interest. Absorption and therefore
the systemic availability remains the exception (Fig. 1 b). However, the detection
of the release of ingredients from various galenic preparations is comparable
to pharmacological research (11). An important quality criterion is the intact,
natural skin barrier within the stratum corneum (12).
| Fig.
3: Adhesive tape strippings  |
In innovative cosmetic concepts vitamins are regarded as being the most important active agents. This is why from the very beginning the behaviour of oil-soluble and water-soluble vitamins, such as vitamin A palmitate, vitamin E and D-panthenol, has been of primary interest. The penetration of substances starts with the migration into the stratum corneum consisting of the horny cells and the lipid double layer between the cells (brick / motar concept). After a dry wipe-off the Emulsion's surplus it is very easy to investigate by using the adhesive tape stripping method. When the tape is torn off the stratum corneum is more or less removed layer by layer except around the hair follicle. As a result of the size of the strips (19 mm * 100 mm) the amount per strip is approx. 1 mg horny layer. The applied test substance is determined by chemical analysis in the lamella and fragments which remain attached to the strip.
Fig.4 shows the results of such a study in the detection of vitamin A palmitate (0.1 %) and vitamin E (RRR-alpha-tocopherol, 0.1 %) from a glycerol-water mixture after a single application and an exposure period of 5 hours (5). The differing penetration pattern, documented by the amount / strip in the various layers can be clearly recognized. Vitamin A palmitate could only be detected as an ester an the first two strippings but not an the succeeding eight strippings. Under these conditions it exhibits a high substantivity which does not lead to further penetration. On the other hand, vitamin E could be detected almost uniformly an all ten strippings and, in contrast to vitamin A palmitate, it penetrates the stratum corneum. An end to the penetration process after 10 strippings could not be recognized.
By the use of dermatome sections the penetration in the viable compartments of the skin could be followed further, as the comparison between the W/O cream and an oil solution demonstrates in exemplary fashion in Fig. 5 (13). The accumulated amount of vitamin E was higher after application of the W/O cream by a factor of three than after the use of the oil solution. The reason for this is the higher thermodynamic activity of vitamin E in the W/0 emulsion. It is not the weighed-in total concentration which is decisive, but rather the effective concentration of active agent in the solution phase. In the uppermost 10 skin sections, i.e. down to a skin depth of 200 µm, the natural vitamin E content of the skin is lower by orders of magnitude than the amount added from outside by application of the oil solution or W/O cream.
| Fig.
4: Absorption of Vit E in W/O emulsion vs. oil solution 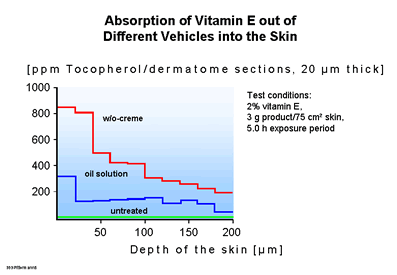
|
In this study further investigations were carried out into the skin penetration properties and emulsion structure using vitamin E and vitamin A palmitate (oil soluble) and D-panthenol (water soluble) as examples in a lamellar O/W and a PIT O/W cream, among others (Tab.1). In 10 stratum corneum strips of skin treated with the PIT cream significantly more of the oil-soluble vitamins were bound after an exposure time of 5 hours than after treatment with the lamellar O/W cream. With panthenol no difference was observed.
The analysis of the skin sections showed a somewhat different picture. In this case the structure of the O/W emulsion had practically no influence an the penetration of D-panthenol or vitamin E. This surprising result indicates that when the PIT emulsion is used the stratum corneum acts as a reservoir for the oil-soluble vitamins. In general the amount of the water-soluble panthenol in the skin is higher than that of the oil soluble vitamin E. The applied amount of 400 ug/cm2 was the same for both vitamins. After 5 hours 30 - 35% of the panthenol was measured in the upper skin layers (to a depth of 200 pm), while for vitamin E only just under half of it was found.
An other type of penetration studies investigating different skin penetration properties of cosmetic formulations under leave-on and rinse-off conditions using the BUS model was published recently (14).
The detection of mucous membrane penetration, e.g. of vitamin E from preparations containing surfactants, is also possible by using dermatome sections or the perfusate. In this case 5.0 ml of the test substance is applied via a apical orificium. After a defined application period the mucous membrane is removed and dermatomized as the skin is prepared for analytical purposes (15).
| Table
1: Penetration behaviour of oil- and water-soluble vitamins in lamellar and PIT
O/W emulsion 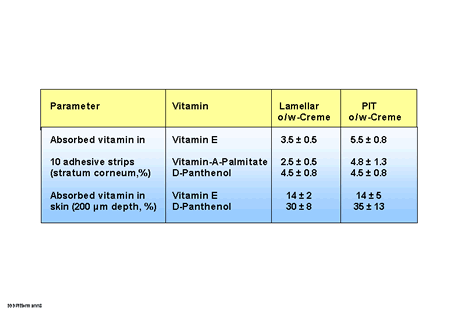
|
Irritation
studies in the skin and mucous membranes
The biological basis for the biochemical investigation of irritation is the intact skin metabolism, which is only possible if skin viability is maintained and proved. For the irritation study punched biopsies (D=6mm) of the skin or mucous membrane were usually prepared after two or more exposure times. This means that is was possible to follow the irritation effect as a function of the penetration ability of the substance. Due to the different cell densities in the tissues the samples were adjusted to a comparable DNA content.
A differentiation is made between cytoxicity and irritancy. The cytotoxicity is determinated by a modified MTT- assay. A measure for cell irritancy is the amount of concentration of prostaglandin E2 or other pre-inflammatory mediators in comparison to untreated tissue. This discrimination of the different pathogenesis between the primary and secondary skin damage is necessary as cytoxicity (MTT) and irritancy (PGE2) are substance-specificproperties. Furthermore the differentiation increases the value of in vitro assays significantly. Extensive investigations, e.g. with mucous membrane antiseptics, have confirmed the different pathophysiological mechanisms (16).
An
important target in the development of new surfactants or surfactant based products
containing surfactants is that they do not irritate the skin or mucous membranes
during the intended use on human beings. This means that irritation tests an the
skin (open and occlusive application) and mucous membranes belong to the basic
investigations carried out. The following example shows a comparison between APG
(alkylpolyglycoside) and SDS (sodium dodecyl sulphate) at concentrations of 3%
and 10% after exposure times of 1 and 5 hours with occlusive application (17).
| Fig.
5 a MTT 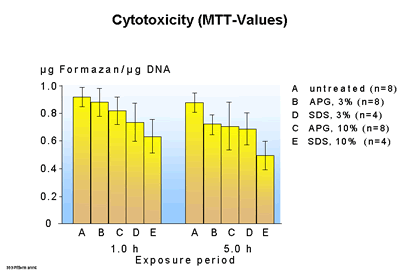
|
| Fig
5b PGE 
|
With regard to cytotoxicity (Fig. 5a) the APG (3, 10%) did not differ appreciably from untreated skin after an exposure time of one hour, while with SDS the concentration of 3% and, above all, the 10% concentration showed a clear difference. After the skin is exposed for a further 4 hours the APG (3, 10%) and SDS 3% become similar, but there is a further increase in the damage caused by SDS 10%, i.e. the number of viable cells whose mitochondria could manage the biochemical conversion of the offered colorant (Methyltetrazolium) is reduced further as skin penetration of the surfactant increases. Similar results are also obtained from the measurement of the prostaglandin E2 - concentration (Fig. 5b). In contrast to reference surfactants APG is required to be used at a very high concentration in order to induce a reduction in tissue compatibility. Surfactants such as alkypolyglycosides therefore offer more formulation possibilities in a wide range of body care products without appreciably altering their skin and mucous membrane compatibility.
It is also possible to compare formulated and market products with one another in the same manner as that used for these surfactant ingredients, no matter whether in open or occlusive application, under leave an or rinse-off conditions (15).
Summary
Many years of experience in research projects in the pharmacological and cosmetics industries have shown the isolated perfused bovine udder (Bovine Udder System, BUS) to be a versatile, economical and scientifically interesting in vitro method. Experimental set ups which have their targets as being the penetration and irritation of skin and mucous membranes by raw materials and finished products to the same extent are at the focus of attention.
Literature
1)
Zbinden, G., Menschen, Tiere und Chemie; M.T.C. Verlag Zollikon (Switzerland),
1985
2) Pittermann, W.; In-vitro- Methoden und Alternativen: Erfahrungen und
Argumente; Kosmetikjahrbuch, 422-433 (1997)
3) Spielmann, H.; Nicht mit Tieren:
Toxikologische In-vitro-Prüfung von Kosmetika; Pharmazeutische Zeitung 143, 11-15
(1998)
4) Kietzmann, M., Arens, D., Löscher, W. and D. Lubach;
Studies an the percutaneous adsorption of dexamethasone using a new in vitro model,
the isolated perfused bovine udder, in: Prediction of Percutaneous Penetration.
R. C. Scott, R. H. Guy, J. Hadgraft and H. E. Boddé (eds.) IBC Technical Services
Ltd: London, (1991) 519-526
5) Jackwerth, B., Pittermann, W. und M.
Kietzmann; Bovine Udder Skin model (BUS): Innovatives In-vitro-Modell zur Penetration,
Resorption und Irritationswirkung kosmetischer Stoffe und Formulierungen; Parfümerie
und Kosmetik 77, 37-40 (1/1996)
6) W. Pittermann; Mit Tierorganmodellen versuchstierfrei
forschen; Parfümerie und Kosmetik 79, 46-47 (11/1998)
7)
Kietzmann M., Löscher W., Arens D., Maaß P. and D. Lubach; The Isolated Perfused
Bovine Udder as an in Vitro Model of Percutaneous Drug Absorption. Skin Viability
and Percutaneous Absorption of Dexamethasone, Benzoyl Peroxide and Etofenamate;
J. Pharm. Toxicol. Meth. 30, 75-84 (1993)
8) W. Pittermann, H. Jungmann,
M. Kietzmann, M. Schmitt, B. Blume; Sichtbare und UV-Spektroskopie am isoliert
perfundierten Eutermodell; Kosmetische Medizin 19, Edition 3, July/August 1998,
152-162 (1998)
9) Beuscher, N., Kietzmann, M., Bien, E. and P. Champeroux;
Interference of Myrtol Standardized with Inflammatory and Allergic Mediators.
Drug Research; 49, 985-989 (1998)
10) Blume, B., Kietzmann, M., Möder, M.,
Kränke, P and M. Wahren; Application of Deuterated Benzoyl Peroxide in an in
Vitro Model of Percutaneous Absorption and Dermal Metabolism of Chemical Substances;
in: Synthesis and Applications of Isotopically Labelled Compounds 1997; J.R.
Heys and D.G. Melillo (eds.), John Wiley & Sons Ltd 1998, pp 597-600
11)
Kietzmann, M., Wenzel, B., Löscher, W., Lubach, D., Müller, B.W. and H. Blume;
Absorption of Isosorbide Dinitrate after Administration as Spray, Ointment and
Microemulsion Patch. An In-vitro Study Using the Isolated Perfused Bovine Udder;
J. Pharm. Pharmacol. 47, 22-25 (1995)
12)
Pittermann, W., Gassenmeier, Th., Nieveler, S., Förster, Th., Kietzmann M.; Experimentally
induced epidermal barrier perturbation: Measurement of trans epidermal water loss
(TEWL) using the isolated perfused bovine udder Skin (BUS) model. IFSCC-Magazine,
Vol. 3/1, 29-32 (2000)
13) Förster, Th., Jackwerth,
B., Pittermann, W., v. Rybinski W and M. Schmitt; Properties of emulsions - structure
and skin penetration; Cosmetic & Toiletries 112, 73-82 (1997)
14)
Förster, Th., Pittermann, W., Schmitt, M. Kietzmann; J. Cosmet. Sci.
Pp 147-157 (1999) Skin penetration properties of cosmetic formulations using a
perfused bovine udder model
15) Pittermann, W., Kietzmann,
M., Krächter, H.-U., Holtmann, W. and A.H. Schoon; The isolated perfused bovine
udder (BUS) model: A new natural in vitro model for mucous membrane compatibility;
in: The Ethics of Animal Experimentation, Proceedings of the EBRA/FELASA European
Congress, Brussels, 1996; Ph. N. O'Donoghue (ed.), Page Bros, UK 1998, pp 265-266
16) Krächter, H.U., Gläser, S. und W. Pittermann; Lokale Verträglichkeit
von Schleimhautantiseptika: Vergleichende Prüfung in einem neuen in-vitro-Modell;
Gynäkologie + Geburtshilfe hautnah 1/1998, 38-42 (1998)
17)
Pittermann, W., Jackwerth, B. and M. Schmitt; The Isolated Perfused Bovine Udder
Skin Model: A New In Vitro Model for the Assessment of Skin Penetration and Irritation;
In Vitro Toxicology 10, 17-21 (1/1997)
German version of this article
was published in Parfümerie & Kosmetik 80, 38-41 (3/1999) and reproduced
in English with permission of the editors.
Author
VITA

Dr. W. Pittermann, Certified
Veterinary Pathologist, Diplomate ECVP,
Henkel KGaA, Duesseldorf, Germany
Born: August 23, 1944 Austria; Study of Veterinary Medicine: Vienna (Austria),
Hannover (Germany)
1969 - 1971: Postgraduate Study for Vet. Pathology and Laboratory Animal
Science (Hannover)
1971: Promotion: DVM (Veterinary School Hannover, Hannover)
1972 - 1977: Basic research (laboratory animal pathology) in a laboratory
animal breeding unit of the German Research Society (Government-owned)
1978: Development of the Laboratory Unit of Toxicological Pathology at
Henkel, Duesseldorf
1983: Responsibility for the laboratory animal unit at Henkel, Duesseldorf
1987 - 1997 Head of the experimental Toxicology and Pathology Unit at
Henkel
1998 -: Laboratory Experimental Dermopathology within the Henkel Research
Group 'Biochemistry Skin'
Main Topics: Toxicological and experimental Pathology, Irritation and Penetration in Skin and Mucous Membranes and Cell Culture, UV-light and Sensitivity Testing, New Technology Since 1985 special experience in in-vitro skin irritation and penetration.
Qualifications:
1975: Certified Veterinary Pathologist
1986 -: Animal Welfare Commissioner at Henkel, Duesseldorf
1993 -1998: Executive Board of the Society of Laboratory Animal Science
1998 -: Scientific Advisory Board (Society of Laboratory Animal Science)
1994 - 1998: Member of the Task Force 'Allergy' (VCI)
1995 - : Deputy Member of the State Animal Welfare Committee
1995: Member: Educational Committee, Society of Toxicological Pathologists
1995 -: Diplomate of European College of Veterinary Pathologist (ECVP)
Copyright
© 2000 - 2026
Institute for Dermopharmacy GmbH
webmaster@gd-online.de
Haftungsausschluss